Safe prescribing of Alemtuzumab in a district general hospital: Sunderland vs Newcastle
2017 MasterClass Project Runner Up
Sunderland Royal Hospital (SRH) is a large district general hospital situated in the North East of England. It serves a population of around 350,000 people and has a small neurology department, consisting of five consultants and two registrars. The multiple sclerosis (MS) service is provided by Dr Kate Petheram, consultant neurologist, and Sister Barbara Wingrove, MS nurse consultant. Fourteen miles to the north, lies the Royal Victoria Infirmary (RVI) in Newcastle upon Tyne, which is the regional neurosciences hub. It has 20 consultant neurologists, including three MS specialists and 4 MS specialist nurses.
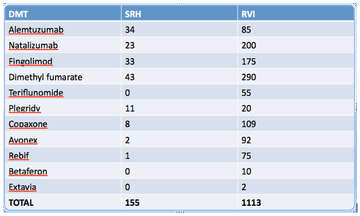
The total number of patients ever registered to have MS on the SRH database is 868. The number of patients on each disease-modifying therapy at SRH compared to the RVI, as of January 2017, is as follows:
Aims
The aim of this project was to audit the provision of Alemtuzumab at SRH and compare it to the RVI. This was prompted by recent Association of British Neurologists guidelines on prescribing disease modifying treatments in MS [1]. These state:
“Our view is that an effective and safe multiple sclerosis team, whether based at a Regional Neuroscience Centre or a District General Hospital, should include more than one MS specialist consultant neurologist” (my italics)
Given there is only one MS specialist consultant neurologist at SRH, we wanted to ensure screening and monitoring of Alemtuzumab was meeting appropriate standards, and was comparable between the two sites.
Standards
Standards for the audit were mainly based upon the summary of product characteristics for alemtuzumab (Lemtrada) [2] and were as follows:
Screening: the following should be performed/addressed prior to treatment:

Monitoring: the following monitoring tests should be undertaken until 48 months after the last course:
- Full blood count (FBC), creatinine, urinalysis with microscopy (monthly)
- Thyroid function (three monthly)
MRI monitoring was based on local standards. Ideally, patients should have:
- A baseline MRI head three months prior to/after start date (and at least in the year prior to treatment)
- An annual follow up MRI head scan (ABN guidelines also suggest consideration of this [1])
Methods
I reviewed the electronic records of all patients on alemtuzumab at SRH up to November 2017 using a proforma. I compared this to monitoring data from the RVI from a sample of 31 patients on alemtuzumab (screening and MRI data not available from RVI).
Results
There were 29 patients on alemtuzumab at SRH. They had similar demographics to the 31 patients at the RVI. Most patients (18 at SRH, 19 at RVI) were treatment naïve. For those who had been on previous treatment, this was most commonly natalizumab (with fingolimod bridge) at both sites.
Screening results:
Screening result from SRH are summarised in table one. All screening items were carried out in 90% or more of patients. 100% of screening blood tests were performed.
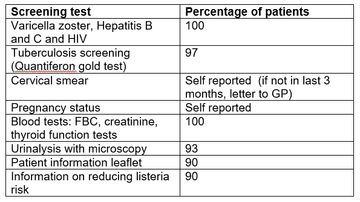
Table 1: Screening results from SRH
Monitoring results:
The results of monitoring of FBC, creatinine, thyroid function (TSH) and urinalysis are shown in figures one to four. Most patients at SRH had 100% of their blood monitoring completed. This was significantly better than the results from the RVI. Most patients had 80% or more urinalysis performed, again, outperforming the RVI.
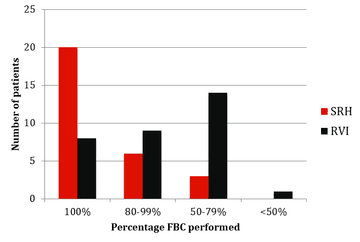
Figure 1: FBC monitoring results from SRH and RVI
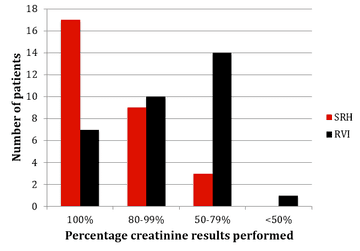
Figure 2: Creatinine monitoring results from SRH and RVI
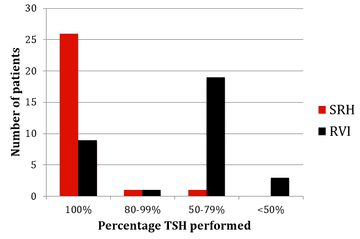
Figure 3: TSH monitoring results from SRH and RVI
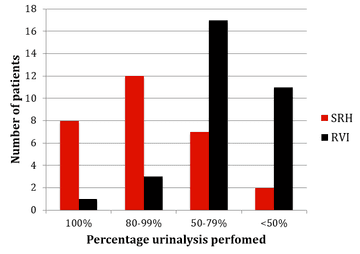
Figure 4: Urinalysis monitoring results from SRH and RVI
MRI results:
Around half of patient (14/29) had a baseline MRI head +/- 3 months from starting alemtuzumab at SRH. All had one in the year prior to treatment. Almost all had a follow up annual MRI post-treatment (9/10) (the one patient who hadn’t, had a scan at 6-12 months).
Discussion
The positive aspects of the results are that the screening and monitoring was very comprehensive in SRH, which outperformed the RVI. Baseline and follow up MRI scanning was also comprehensively performed.
Some minor points that could be improved upon are that a significant number of urinalysis tests were missed and that pregnancy and smear results rely on patient self-reporting (although information about this is provided in the patient information pack). All patient had an MRI in the year prior to treatment. However, more patients could have one +/- 3 months of starting, rather than at 6-12 months, as this gives less useful information, being neither a baseline scan or an annual follow up scan.
Some limitations of the audit were that I only used electronic records to gather information and that I only had limited information from RVI.
The results are very reassuring in terms of the safe provision of alemtuzumab at SRH. We are meeting the vast majority of screening and monitoring standards, and, indeed, outperformed the RVI in terms of monitoring, which has a larger MS team.
Some potential reasons for this are that SRH is a smaller centre with less patients on alemtuzumab. Therefore, a MS specialist consultant and MS nurse consultant can manage all the patients in a centralised service, providing more personalised care and managing all the blood monitoring personally (compared to outsourcing some of it to GPs at the RVI). There seems to be no problem accessing MRI scanning, and this may be because SRH is a smaller centre with no competing demands from, for example, neurosurgery.
A major potential disadvantages of the service at SRH is that it is precarious. If either the consultant or nurse consultant were to become unavailable, then this would leave a large number of patients on alemtuzumab without specialist cover. Another disadvantage is that there are no other MS consultant colleagues on site to discuss cases. However, there is a monthly regional MS meeting at the RVI for this purpose.
Action plan
- Department to review timings of MRI scans
- Currently setting up DAWN blood monitoring software
- Business plan for second MS specialist nurse (and possibly second MS specialist consultant)
References
- Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Scolding N, et al. Pract Neurol 2015;0:1–7.
- Electronic Medicines Compendium (2016). “Summary of product characteristics – Lemtrada”. Available at: https://www.medicines.org.uk/e... [accessed February 2017].
More MS Academy Medication Projects
Encouraging excellence, developing leaders, inspiring change
MS Academy was established five years ago and in that time has accomplished a huge amount. The six different levels of specialist MS training are dedicated to case-based learning and practical application of cutting edge research. Home to national programme Raising the Bar and the fantastic workstream content it is producing, this is an exciting Academy to belong to.